On June 3, 2015, the United States Food and Drug Administration issued its final rule amending the veterinary feed directive drugs section of the Animal Drug Availability Act of 1996. The aim of the amendment is to ensure the judicious use of medically important antimicrobials in food-producing animals. Approved uses are to treat animals diagnosed with an illness, control the spread of illness in a herd, and prevent illness in healthy animals when exposure is likely.
What does this really mean? Antibiotics used in animal feeds which are also prescribed in humans will require a veterinary feed directive (like a prescription) from a veterinarian. These medications include: Penicillins, Cephalosporins, Quinolones, Fluoroquinolones, Tetracyclines, Macrolides, Sulfas, Glucopeptides, and others. Vets may only approve the use of those feeds to treat, control, or prevent disease. The FDA is concerned that overuse in animals may reduce the effectiveness in humans. The purpose of the veterinary feed directive is to promote judicious use of such medications in livestock feed. Their aim is improve the efficiency of the VFD process, streamline administrative procedures, and provide veterinarians with greater flexibility.
The implementation of the rule is voluntary until January 1, 2017. Voluntary compliance allows producers, dealers, and feed mills to make all the necessary changes needed in their own time, so that they are fully prepared by the first of next year. Once all labels have been transitioned, compliance will no longer be voluntary.

For Producers
Most livestock owners want to know how they can keep their livestock healthy, without violating the law. First, it’s important to understand that medicated feeds will only be allowed for disease treatment, control, and prevention. If you feel you need a medicated feed, you must call your veterinarian for a VFD.
Does this mean your veterinarian has to come out to your operation every time you need a medicated feed? Not necessarily. The veterinarian can issue a VFD without seeing the specified animals if they meet the following requirements:
- Engage with the client to assume responsibility for making medical judgements about animal health and the need for medical treatment.
- Have sufficient knowledge of the animal by virtue of examination and/or visits to the facility where the animal is managed to initiate at least a general or preliminary diagnosis of the medical condition of the animals
- Provide for any necessary follow-up evaluation or care
Basically, as long as the veterinarian has a relationship with you and is familiar with your operation, they can issue VFDs without necessarily seeing the animals every time.
You then take that VFD in to your dealer to purchase the feed. You must keep all your VFD records for a minimum of two years. How much feed you purchased, how long you fed it, and to how many animals.
There are two important parts of the VFD that you must pay close attention to: the expiration and the duration.
The expiration date defines the amount of time the dealer will be allowed to sell you the medicated feed. You may continue to feed the feed up until the expiration date. However, once that expiration date passes, you must get another VFD in order to continue feeding the medicated feed.
The duration determines the length of time the VFD feed is allowed to be fed to the animals. It will be specified on the product label. For example, a five day feeding limit, with a 15 day rest period in between use (these durations may vary depending on the drug, and have not been determined, yet).
For Dealers
Prior to selling any VFD medicated feeds, you must send a one-time distributor notification to the FDA. This statement lets the FDA know that you plan on distributing VFD feeds. It must include the following:
- The distributor’s complete name and business address
- The distributor’s signature or the signature of the distributor’s authorized agent
- The date the notification was signed
|
This statement should be sent to: Food and Drug Administration Center for Veterinary Medicine Division of Animal Feeds (HFV – 220) 7519 Standish Place Rockville, MD 20855 Or you can fax it to: 1-240-453-6882 |
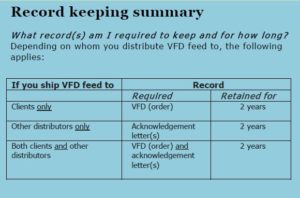
You also must send an acknowledgement letter to every manufacturer that provides you with medicated feed products. The acknowledgement letter informs the manufacturers that you understand and will comply with all VFD requirements. It can either be hard copy, or sent through e-mail, and must also affirm:
- That you will not ship such VFD feed to an animal production facility that does not have a VFD
- That you will not ship VFD feed to another distributor without receiving an acknowledgement letter
- That you have sent the FDA your distributor notification
All VFD forms will come from the veterinarian, in triplicate. A copy for the vet’s records, a copy for your records, and a copy for producer’s records. You must maintain records of the receipt and distribution of all medicated animal feed containing a VFD drug for a minimum of two years, as well as copies of the VFDs. The FDA may request an inspection of these records at any time. If you sell VFD feed to another distributor, they must send you and acknowledgment letter, and these must also be kept and available for inspection.
It will be your responsibility to ensure the VFD contains all the required information, and you must not sell the product after the expiration date. You also need to ensure that all labelling and advertising prominently displays the following statement: “Caution: Federal law restricts medicated feed containing this veterinary feed directive (VFD) drug to use by or on the order of a licensed veterinarian.”
It would be a good idea to develop a relationship with the veterinarians in your area so that they are aware of what products you have, and what products can be obtained if needed. A good working relationship with area veterinarians will help streamline the VFD process, and ensure thorough communication for all parties involved.
For more information, visit the FDA web page on the Veterinary Feed Directive. You can also visit: http://www.fda.gov/safefeed. If you have any additional questions, don’t hesitate to call your Hi-Pro representative.
*Source for graphics – FDA “Requirements for Distributors (Who do not manufacture VFD feed)” brochure, and “Requirements for Producers” brochure.


